Monday, April 25, 2011
Protein in Monkeys Makes Them Resistant to HIV
Sunday, April 24, 2011
The particles are described as having a nanoporous core with a high surface area and an encapsulating lipid bi-layer (liposome). The nanoparticles and the surrounding cell-like membranes formed from liposomes together become the combination referred to as a protocell: the membrane seals in the deadly cargo and is modified with molecules (peptides) that bind specifically to receptors overexpressed on the cancer cell's surface. (Too many receptors is one signal the cell is cancererous.) The nanoparticles provide stability to the supported membrane and contain and release the therapeutic cargo within the cell. The lipids also serve as a shield that restricts toxic chemotherapy drugs from leaking from the nanoparticle until the protocell binds to and takes hold within the cancer cell. This means that few poisons leak into the system of the human host, if the protocells find no cancer cells. This cloaking mitigates toxic side effects expected from conventional chemotherapy.
This method is currently being tested on human cells in vivo (occurring or carried out in a living organism) and will shortly be tested in mouse tumors. Estimates are that this method will be 10,000 times more effective than current liposome delivery methods and may be available in as early as 5 years. This will be the first work to show targetted delivery of nanoparticles to cancers supported in part by a grant from the National Cancer Institute's Alliance for Nanotechnology in Cancer .(1)
This method provides hope for easier treatment of some cancers. As well, the specific targetting that is being attempted will reduce the side effects of cancer treating drugs in the patient. This may lead to better long term health for the cancer survivor as well as less painful treatments.
1. Carlee E. Ashley, Eric C. Carnes, Genevieve K. Phillips, David Padilla, Paul N. Durfee, Page A. Brown, Tracey N. Hanna, Juewen Liu, Brandy Phillips, Mark B. Carter, Nick J. Carroll, Xingmao Jiang, Darren R. Dunphy, Cheryl L. Willman, Dimiter N. Petsev, Deborah G. Evans, Atul N. Parikh, Bryce Chackerian, Walker Wharton, David S. Peabody, C. Jeffrey Brinker. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nature Materials, 2011
Friday, April 22, 2011
Salmonella Utilize Multiple Modes of Infection

According to the World Health Organization, the number ofSalmonella infections is continuously rising, and the severity of infections is increasing. One of the reasons for this may be the sophisticated infection strategies the bacteria have evolved. The striking diversity of invasion strategies may allowSalmonella to infect multiple cell types and different hosts. Salmonella do not infect their hosts according to textbook model. Only a single infection mechanism has seriously been discussed in the field up till now -without understanding all the details.
Lyme Disease
As early as the tick bite itself, Borrelia burgdorferi can bypass the immune system in several ways. The tick has several agents in its saliva that coat the invading spirochetes, protecting them as they enter the body through the skin. This allows the bacterium to go unrecognized as a foreign invader. Therefore the immune system goes without "seeing" them. The immune system of someone infected with Borrelia burgdorferi may go for weeks without producing antibodies. Borrelia has flagellum which enables it to invade tissues and thick mucous that normally most other bacteria would not be able to invade. The flagellum excites the immune system. The immune system then recognizes the bacterium is present and responds by producing antibodies. Borrelia is able to go through metamorphosis by changing its proteins on its outer cell wall and the immune system cells are not able to recognize the bacteria. The immune system typically uses cell wall proteins to detect a foreign invader, and develop specific antibodies to mount a coordinated immune attack. As a result of the transformation of the spirochete the immune cells know the bacterium is there but the bacterium is disguised to fit in. The immune system sends in all available immune cells to destroy everything in the area, and in consequence body tissue is destroyed.
References:
http://www.marvistavet.net/html/body_lyme_disease.html
http://www.unmedu/altmed/lyme-disease-000102.htm
http://www.lymediseaseblog.com/how-lyme-disease-affects-immune-system/
Tick Pathogens

In the United States, some ticks carry pathogens that can cause human diseases. Not all ticks carry a disease or are harmful, but if one that has the tick borne disease should bite you it can have a broad degree of severity in humans. Ticks feed on many vertebrates such as dogs, medium sized mammals and small rodents. The two most common species of tick vectors in the United States are the American dog tick, and the Rocky mountain wood tick.
In North America, Rickettsia rickettsii is transmitted by the American dog tick, and the Rocky mountain wood tick. R. Rickettsii is a rod shaped bacterium known to cause Rocky Mountain Spotted Fever. Ticks are infected with R. Rickettsii while feeding on blood from the host in the larval, nymphal or adult stage. Once the tick gains this pathogen from its host, they remain infected for life. After an immature tick develops into the next stage of its life it can be passed on to the secondary host.
Ticks perch in low vegetation and wait for a susceptible host on which they can attach and feed on. Various online sources describe how ticks enter a host cell. First the R. Rickettsii attach to a protein-dependent receptor on the cell membrane of the host. The cell wall of R.Rickettsii is composed of the outer membrane, peptidoglycan, and cytoplasmic membrane. This makes it hard to stain with the Gram stains and view under the microscope. Secondly, with the aid of the outer membrane Protein A (ompA), the adhesion of this molecule to the host cell induces the local cytoskeleton arrangements with the cell, which results in their entry into the cell. Once a tick attaches to its host, some are known to secrete a cementing material to fasten themselves to the host. Some ticks secrete an anticoagulant, immunosuppressive, and anti inflammatory substance into the area of the tick bite to help the tick obtain a blood meal without the host noticing. The same substances help any freeloading pathogens to establish a foothold in the host.
The Damage following R. Rickettii happens in the blood vessels of the human body, mostly in the brain, skin, and the heart. The bacteria are able to live in the cytoplasm of the nucleus of the host cell. When that bacteria replicate, the results are severe damage and often death of the cells in which it lives in. During multiplication, blood leaks out into nearby tissues through holes in the vessel walls. This obstructs the flow of blood. This part of the cycle involving injury to the blood vessels causes the rash associated with Rocky Mountain spotted fever, in addition to other symptoms including stupor and terminal shock. Death is often caused through excessive damage to the endothelial cells, resulting in the leaking of plasma, decrease in blood volume and shock.
Ticks can transmit many pathogens such as; bacteria, spirochetes, Rickettsia, protozoa, viruses, nematodes and toxins. Tick bite contraindications can resemble arthritis, or flulike symptoms, so it is always a good idea to check yourself anytime after a camping event, or if you have animals that may carry them on their fur. Taking the time to check yourself can prevent a wide variety of symptoms from something as simple as a rash or to something as dangerous as paralysis, shock, or death. Some bites can be cured with and antibiotic, unless it has invaded the Central Nervous System.
References:
1.) http://microbewiki.kenyon.edu/index.php/Rickettsia_rickettsii
2.)http://www.bio.davidson.edu/people/sosarafova/Assets/Bio307/liwoeste/PathogenLifeCycle.html
3.)http://emedicine.medscape.com/article/786652-overview
Sunday, April 17, 2011
Herpes Simplex Virus Type 2
Data was collected from studies performed by Dr. Anna and her colleagues from 1992 to 2008, of 498 seropositive HSV-2 carriers. Polymerase Chain Reaction was performed on samples from these studies, (collected for 30 consecutive days), of the genital mucous of these individuals for testing of the viral DNA. Subclinical genital shedding rates along with the PCR testing revealed that "...the median [midpoint] amount of HSV detected ...was similar in persons with symptomatic and asymptomatic infection." The same studies also revealed that those individuals presenting lesions (symptoms) were indeed infectious while symptoms lasted (43% of the total amount of days they were tested for, carriers were shedding), but asymptomatic persons only presented 16.4% of the the time (in testing.) These findings demonstrated that asymptomatic individuals were still at risk for "shedding" or passing on the infection, despite an absence of visible lesions.
Although it is not fully understood how the herpes virus invades cells, it is understood that "most viruses need cell-entry proteins called fusogens in order to invade cells," but herpes requires fusogens plus two other "entry" proteins to invade cells. Efforts to combat the disease are ongoing, and research and experiments performed by many scientists including those of Dr. Ekaterina Hedwein have "led us to believe that this protein complex is not a fusogen itself but that it regulates the fusogen. We also found that certain antibodies interfere with the ability of this protein complex to bind to the fusogen, evidence that antiviral drugs that target this interaction could prevent viral infection."
As many already know, there is no cure for the herpes virus and it persists in the body for the infected person's lifetime, retreating or resurfacing at random and irregular intervals. Known effects of the virus range from cold sores, encephalitis, blindness, cancer, and in cases of transmissions from mother to fetus, even death (of the fetus). Hence, despite what we think we already know about risks of transmission and keys to avoiding infection, ongoing research is imperative to finding a cure for those who, for one reason or another, have contracted the virus
insciences.org/article.php?article_id=9255
http://www.sciencedaily.com/releases/2011/04/110412101324.htm
lib.jiangnan.edu.cn/ASM/385-2.jpg
Toothbrush Bacteria
Have you ever wondered what it would be like to only have to brush your teeth once a week or even once a month? Well, scientists have identified an enzyme in the human mouth that prevents the buildup of plaque which inevitably leads to tooth decay. The interesting part of this is that this enzyme is naturally produced in our mouths.
The human mouth is full of bacteria that are helpful to our well-being. These bacteria break down the food we eat, keep our mouths clean, and fight of certain infections. The main bacteria that cause plaque, Steptococcus mutans, produce acid from the sugars that we eat. This acid eventually wears down the main protective covering of our teeth known as enamel. Once the enamel is gone, there is no coming back. Conveniently, this is not the only species of bacteria that inhabit our mouths. The good kind, S. salivarius, inhibit the buildup of the bad kind of bacteria, S. mutans.
Through specific tests and careful observations, scientists were able to detect what exactly caused the “good” bacteria to inhibit the spread of the “bad” bacteria. What they found was that a certain enzyme known as, FruA, breaks down sugars thus preventing the buildup of plaque. With these findings, people may believe that they can eat all the candy they wants, but that is not the case. Sucrose, the sugar most commonly ingested by humans, was shown to prevent the “good” bacteria from inhibiting the bad bacteria.
Now, all these scientists have to do is implement this enzyme into toothpastes. The only problem they have is developing a way to keep the enzyme active on the shelf like toothpastes available now. They do believe that the recent information regarding these enzymes will lead to the development of better toothpaste. When they develop these new toothpastes, dentists might start going out of business because no one will need them again!
http://news.sciencemag.org/sciencenow/2011/04/a-bacterium-that-acts-like-a-toothbrush.html
Images:
http://microbewiki.kenyon.edu/images/thumb/5/52/26643C.jpg/250px-26643C.jpg
http://www.orientalcures.com/Images/ToothDecay.jpg
Proteus Vulgaris

Is Your Meat Contaminated?

Sunday, April 10, 2011
Helicobacter pylori: The Cause of Peptic Ulcers
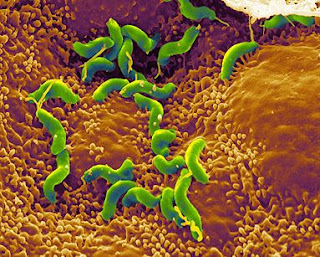 Approximately one-sixth of the world's population suffers from stomach (peptic) ulcers caused by the hard to treat microbe, Helicobacter pylori (H. pylori). A peptic ulcer is a sore on the lining of the stomach or the duodenum. A common misconception of ulcers are that they come from stress or eating spicy foods, but it is H. pylori that cause these painful sores. The bacterium causes peptic ulcers by damaging the mucous coating that protects the stomach and duodenum. When the mucous coating is damaged it then allows stomach acid to get to the sensitive lining beneath. H. pylori is thought to be obtained through food that has not be washed well or cooked properly or from drinking water that has come from an unclean source. It is also thought that an infected person can spread the bacterium to an uninfected person. Although researchers are still unclear how this works it is thought that it can be passed by an uninfected person coming in contact with the stool or vomit of an infected person. It is also thought that it can be passed through the direct contact of saliva.
Approximately one-sixth of the world's population suffers from stomach (peptic) ulcers caused by the hard to treat microbe, Helicobacter pylori (H. pylori). A peptic ulcer is a sore on the lining of the stomach or the duodenum. A common misconception of ulcers are that they come from stress or eating spicy foods, but it is H. pylori that cause these painful sores. The bacterium causes peptic ulcers by damaging the mucous coating that protects the stomach and duodenum. When the mucous coating is damaged it then allows stomach acid to get to the sensitive lining beneath. H. pylori is thought to be obtained through food that has not be washed well or cooked properly or from drinking water that has come from an unclean source. It is also thought that an infected person can spread the bacterium to an uninfected person. Although researchers are still unclear how this works it is thought that it can be passed by an uninfected person coming in contact with the stool or vomit of an infected person. It is also thought that it can be passed through the direct contact of saliva. Electric Microbes For Alternative Energy Use

http://www.newscientist.com/article/dn19804-life-electric-microbes-wire-up-to-share-energy.htm
http://electricmicrobe.com/2010/11/27/how-do-electric-microbes-work/
http://www.sciencedaily.com/releases/2009/07/090729210821.htm
Thursday, April 7, 2011
 Food safety is a major focus of food microbiology. Pathogenic bacteria, viruses, and toxins produced by microorganisms are all possible contaminants of food. However, microorganisms and their products can also be used to combat these pathogenic microbes. Probiotic bacteria can kill and inhibit pathogens. Also, bacteriophages, viruses that only infect bacteria, can be used to kill bacterial pathogens. Thorough preparation of food, including proper cooking, eliminates most bacteria and viruses. However, toxins produced by contaminants may be heat resistant, and some are not eliminated by cooking.
Food safety is a major focus of food microbiology. Pathogenic bacteria, viruses, and toxins produced by microorganisms are all possible contaminants of food. However, microorganisms and their products can also be used to combat these pathogenic microbes. Probiotic bacteria can kill and inhibit pathogens. Also, bacteriophages, viruses that only infect bacteria, can be used to kill bacterial pathogens. Thorough preparation of food, including proper cooking, eliminates most bacteria and viruses. However, toxins produced by contaminants may be heat resistant, and some are not eliminated by cooking.

Probiotics are living organisms that, when consumed, have beneficial health benefits outside their nutritional effects. There is a growing body of evidence for the role of probiotics in gastrointestinal infections, irritable bowel syndrome and inflammatory bowel disease.3
Lactobacillus species are used for the production of yogurt, cheese, sauerkraut, pickles, beer, wine, cider, kimchi, chocolate and other fermented foods, as well as animal feeds such as silage. In recent years, much interest has been shown in the use of lactobacilli as probiotic organisms and their potential for disease prevention in humans and animals.4
1 Fratamico PM and Bayles DO (editor). (2005). Foodborne Pathogens: Microbiology and Molecular Biology. Caister Academic Press.
2 Tannock GW (editor). (2005). Probiotics and Prebiotics: Scientific Aspects. Caister Academic Press.
3 Ljungh A, Wadstrom T (editors) (2009). Lactobacillus Molecular Biology: From Genomics to Probiotics. Caister Academic Press.
4 Mayo, B; van Sinderen, D (editor) (2010). Bifidobacteria: Genomics and Molecular Aspects. Caister Academic Press.
Genetic Rearrangement in Mice

Monday, April 4, 2011
Cryptococcus: A fungus that loves the sugar in your brain

http://www.sciencedaily.com/releases/2010/04/100405152757.htm
http://www.sott.net/articles/show/206336-This-Is-Your-Brain-on-Cryptococcus-Pathogenic-Fungus-Loves-Your-Brain-Sugar
http://www.google.com/search?hl=en&defl=en&q=define:Cryptococcus&sa=X&ei=y22aTZriDeLX0QHh4YTHBg&ved=0CBQQkAE